A highly contagious bird flu epidemic has spread from continent to continent since 2020. It has killed hundreds of millions of wild birds and caused sporadic outbreaks among poultry. In recent months, the avian influenza strain that causes the disease (H5N1) has jumped to dairy cows, domestic cats, and even humans.
In one case this year, a Missouri patient contracted the disease seemingly without exposure to an infected animal. It’s currently unknown how the patient contracted the virus. Some experts have suggested it may have been from consuming raw milk, but while traces of the virus have been found in unpasteurized milk samples, the limited information available makes it challenging to determine if raw milk is a potential source (though, it should be noted, pasteurized milk is perfectly safe to drink).
While bird flu in humans is a cause for concern, transmission remains fortunately rare. According to the World Health Organization (WHO), only 896 cases have been reported worldwide between January 2003 and July 2024. That said, if cases of bird flu in humans continue to rise, it could mean the virus is getting better at infecting people. In a recent study published in Nature Communications, researchers at the European Molecular Biology Laboratory (EMBL) discovered mutations in the bird flu genome that could give the virus this ability by allowing it to better exploit mammalian ANP32 proteins.
Viruses of a feather (don’t always stick together)
Viruses are incapable of reproducing outside a host. To make more of themselves, they must first invade a host cell and take over its cellular machinery. In the case of the bird flu virus, its polymerases — enzymes that make new copies of the virus genome — interact with ANP32 proteins to form a structure called the replication complex. As the name suggests, this complex is necessary for the virus to replicate. (In fact, in a different study, scientists used CRISPR gene-editing technology to engineer chickens resistant to bird flu. They did so by targeting the genes coding for ANP32 proteins.)
However, differences in avian and mammalian versions of this protein explain why avian influenza doesn’t typically infect mammals. Specifically, the avian ANP32 has an amino acid tail that is missing in human ANP32. Researchers have previously observed that virus particles could hold on to the avian ANP32 as they move from birds to mammals, possibly explaining some of the cases of avian flu in mammals. Even so, for the virus to be more successful in mammals, it must be able to use the mammalian ANP32 as effectively as the avian version.
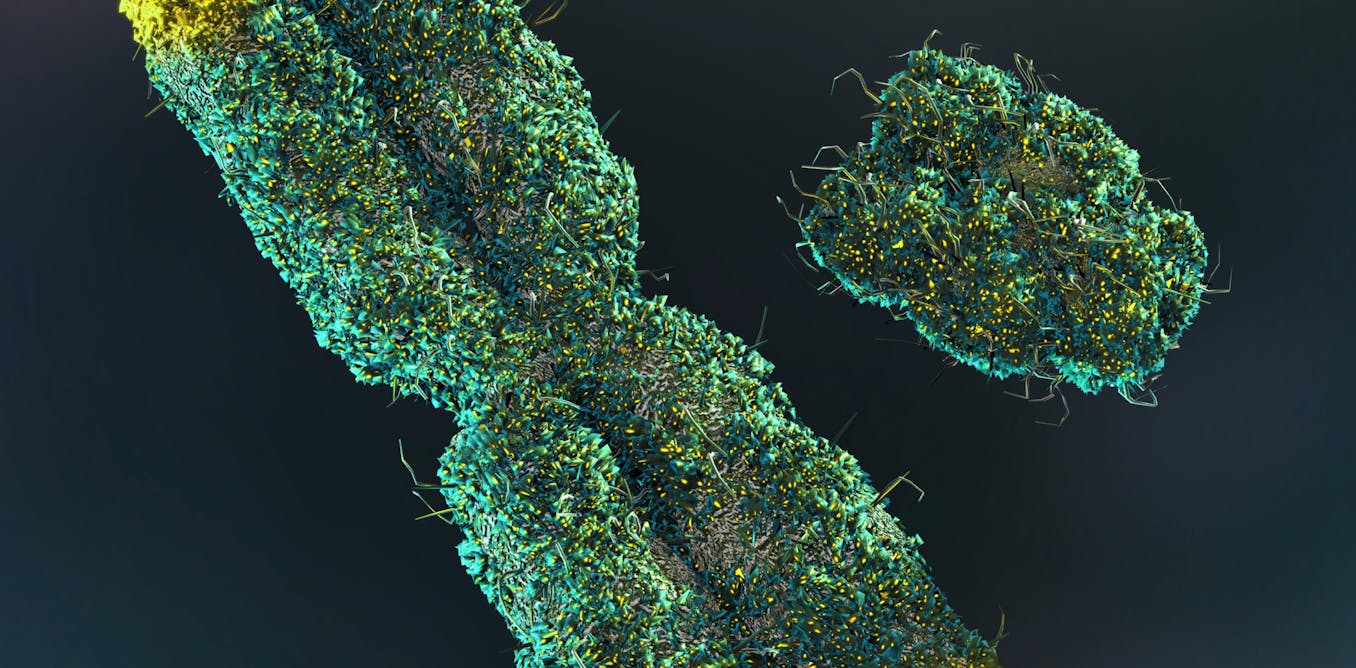
In the recent study, the EMBL researchers used cryo-electron microscopy (cryo-EM), an advanced imaging technique with near-atomic resolution, to look at the structures of the viral polymerases of human-adapted bird flu strains as they interacted with human ANP32.
They found that the ANP32’s amino acid tail steadies the viral polymerases. Besides replicating the viral genome, the polymerases encapsulate the genome into virus particles that subsequently infect other cells. When carrying out the two functions, the polymerases take on two conformations, termed replicases and encapsidases. The study revealed that ANP32 creates a bridge between the two conformations, and this bridge facilitates the assembly of the replication complex.
The extremely detailed cryo-EM structures of the replication complex revealed which individual amino acids participated in the assembly of the replication complex. In other words, the researchers could figure out which amino acids in the replication complex were essential to the replication of the virus. Mutations in these regions of the viral polymerase could make it bind more tightly to human ANP32, potentially improving the virus’s ability to replicate inside human cells.
Keeping better tabs on avian influenza
To monitor the spread of avian influenza, the Centers for Disease Control and Prevention sequences viruses from samples obtained from infected animals in addition to tracking reports of illnesses and deaths. This is to trace mutations in the virus populations. The EMBL study establishes a link between the structure of the replication complex and adaptive mutations in the viral polymerases. Researchers could use this insight to determine if a particular bird flu virus strain is close to gaining the ability to infect people.
As bird flu continues to spread among birds and cattle, there will be more opportunities for the virus to jump to the humans who contact them. This is why experts suggest that more animals, and people around them, be checked for bird flu even in the absence of symptoms. Bird flu becoming the next pandemic is still an unlikely scenario as there is no evidence of human-to-human transmission of the virus.
But while human cases of bird flu may not be widespread, they are highly deadly. Of those 896 cases reported between 2003-2024, 463 were fatal. The high lethality could arguably be attributed, in part, to the fact that many human cases lack obvious symptoms and go under-diagnosed. Regardless, there is a pressing need for therapies to treat human cases of avian influenza as well as stem the spread of avian influenza outbreaks among poultry and cattle.
While there are many influenza virus strains, and new ones keep emerging, the replication complex is central to how they infect and replicate in host cells. Future studies will need to investigate the dynamics of replication in more detail to make the replication complex a key target for anti-influenza drugs.
This article Decoding bird flu: New research reveals potential route for human infection is featured on Big Think.

The post “Decoding bird flu: New research reveals potential route for human infection” by Sachin Rawat was published on 10/19/2024 by bigthink.com
































Leave a Reply